Number of moles formula - How to Calculate and Solve for the Number of Moles, Volume and Molar Concentration in Chemistry
Recent Posts
- Stpm result 2021 release date
- Snapkis car seat
- Bpr 2021 申请
- Ubat bisul paling mujarab
- Pavni reddy
- Jelaskan dua bentuk kesalahan rasuah
- Twenty five twenty one ep 9 eng sub
- Adam toledo
- U dictionary
- Siaran langsung euro 2021 astro
- Bayaran sip prihatin
- The powerpuff girls movie
- Dulu lapar tapi sekarang dah kenyang lyrics
- Resepi bingka ubi cheese
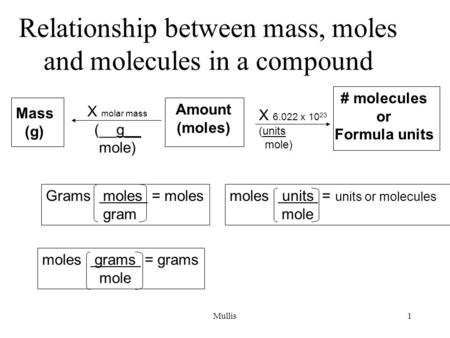
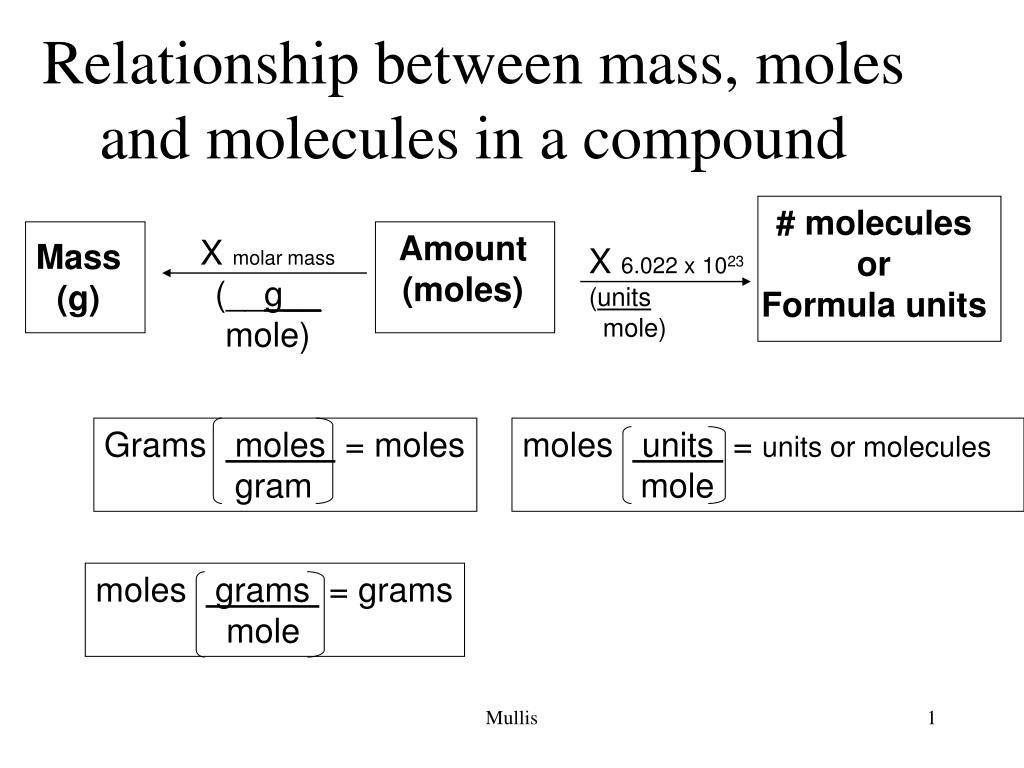
Molecules, Moles and Avogadro's Number
Depending on the nature of the reaction and the substance, the units could be electrons, ions, atoms, or molecules.
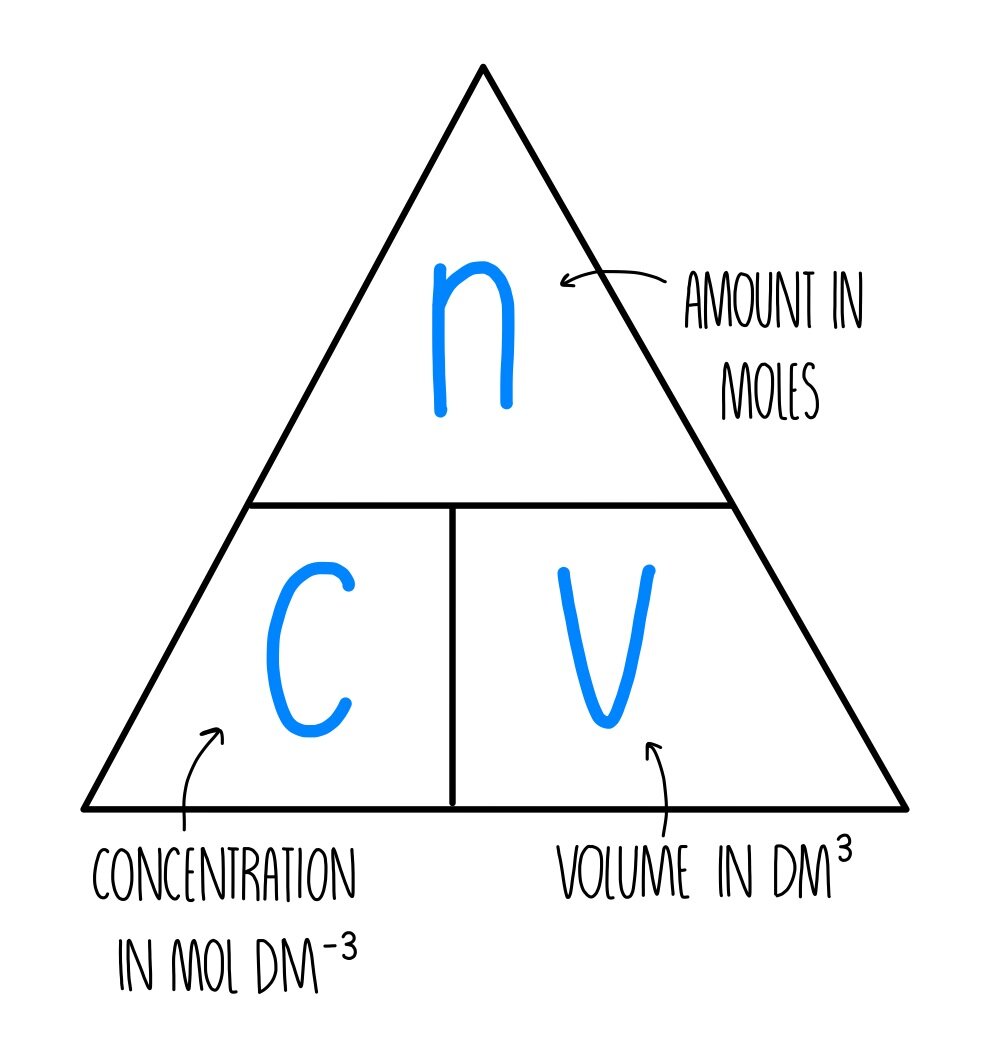
Molarity Calculation It refers to the number of moles per litre of solution.
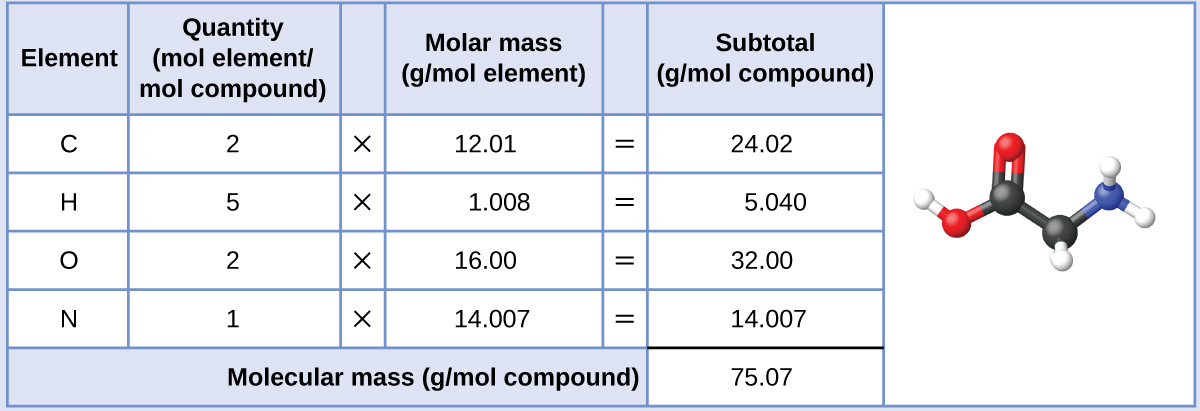
Like even one gram of a pure element comprises a huge number of atoms, which is the reason we are using the mole concept.
How do you convert formula units to moles?
The present definition of the Mole is defined, but it used to be based on the numbers of atoms in a sample of isotope carbon - 12.
Understanding how the mole applies to mass and the number of entities.
Stacked vertically, a mole of pennies would be 4.
- Related articles
2022 mail.xpres.com.uy