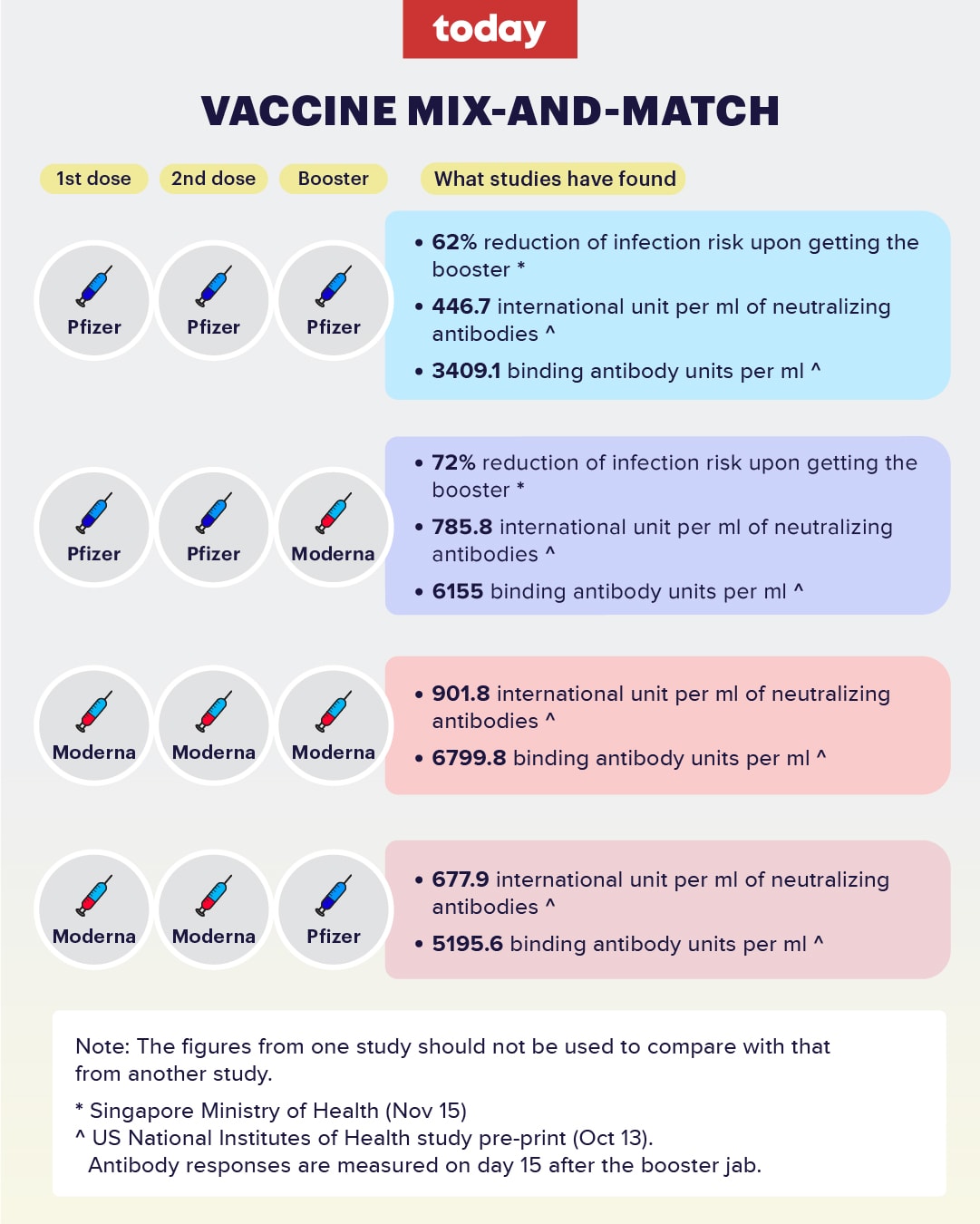
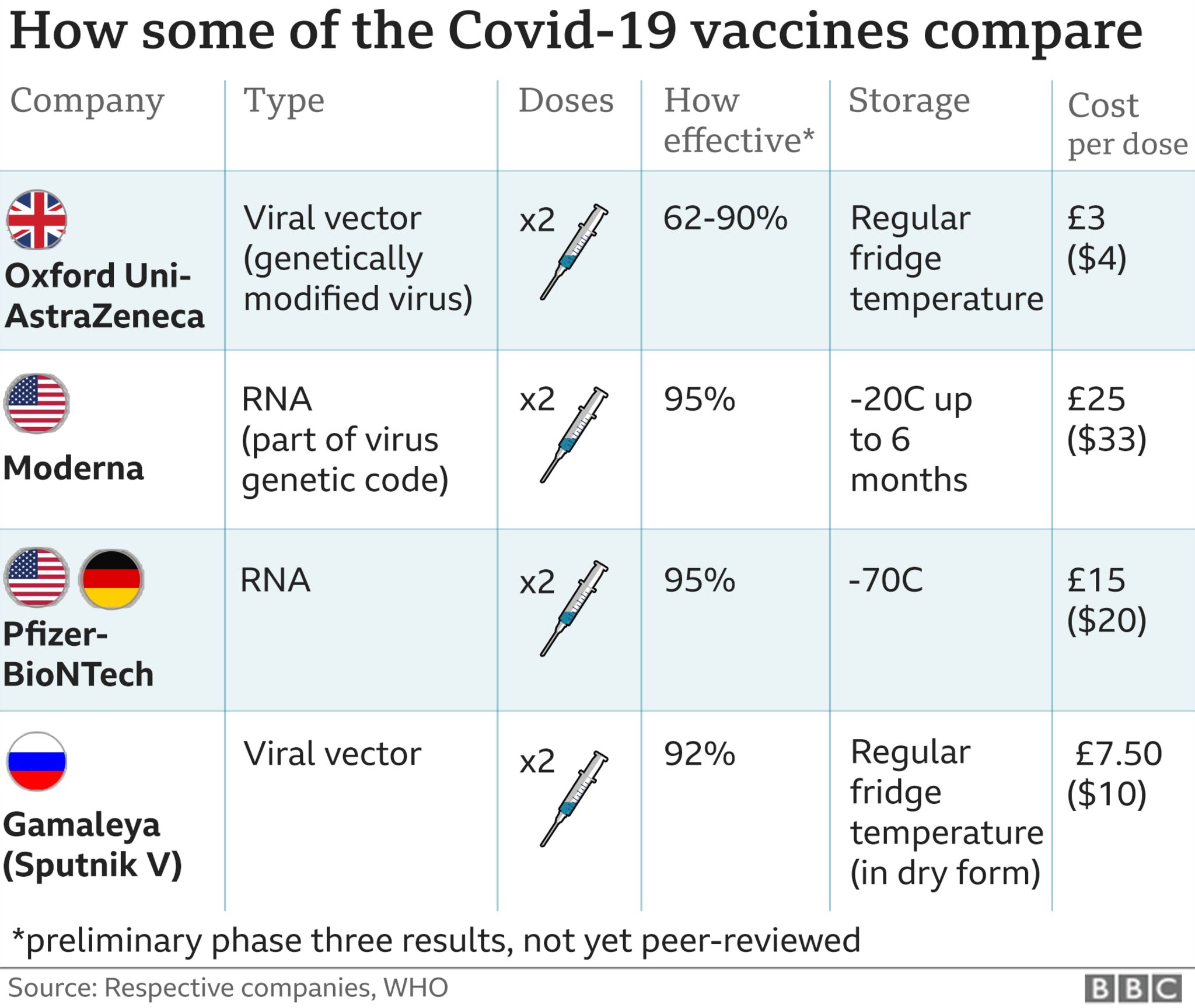
Side effects of sinovac - What are the Sinopharm and Sinovac vaccines? And how effective are they? Two experts explain
CoronaVac COVID
Sinovac was the first company worldwide to receive approval for its H1N1 influenza vaccine.
Efficacy against hospitalisation for Sinovac in Chile, Brazil and Turkey was , respectively.
In Brazil, the immunizing agent against covid-19 is authorized only for those over 18 years of age.
Sinovac (CoronaVac) COVID
The results indicate that a third of CoronaVac® induces a strong immune response in healthy adults with no serious adverse reactions related to the vaccine.
In addition, an article published by the journal on June 4, 2021, stated: "CoronaVac will significantly contribute to the global fight against COVID-19 as a safe and moderately effective SARS-CoV-2 vaccine," says.
The vaccines contain many proteins the immune system can respond to, stimulating the production of antibodies to fight COVID-19.
- Related articles
2022 mail.xpres.com.uy