Hydrogen bond - Hydrogen Bond Examples in Chemistry
Recent Posts
- Kapas 意思
- The beginning after the end light novel
- Iphone 13 pro max wallpaper
- Bangladesh high commission malaysia online appointment
- Cara cek bkc online
- Lux bar
- Isaimini tamil movies 2022
- Kemaskini bantuan keluarga malaysia 2022
- Lee chong wei coach
- Sop kemasukan ke sabah
- Ignis wraith
- Retarder
- Myimms e services fomema
- Semakan keputusan stpm 2021
- Anak mamak menantu mami
- Melayu tudung twitter
- Choi tae joon drama
- The edge of war
- Ucapan selamat menunaikan umrah
- Jnt wakaf bharu
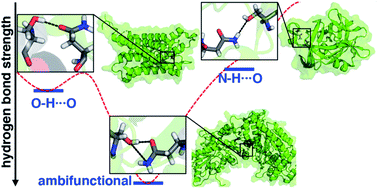
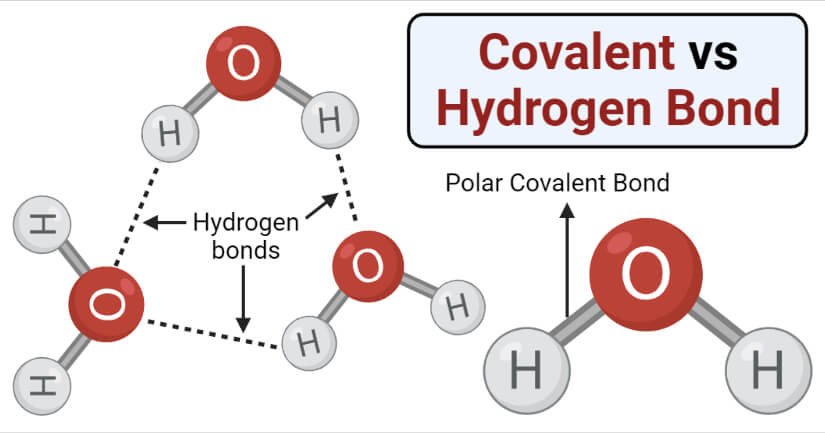
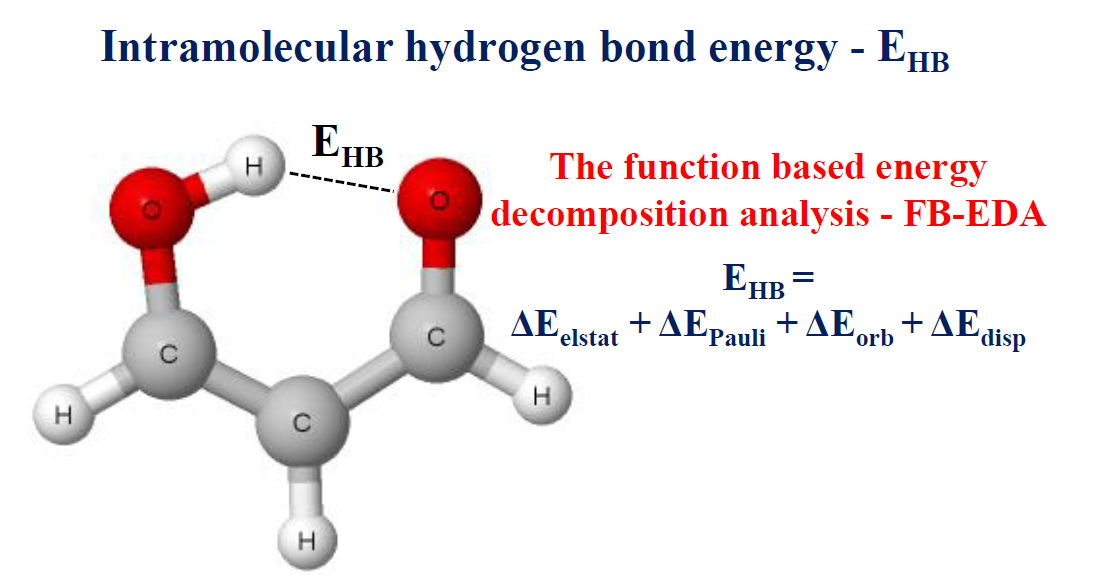
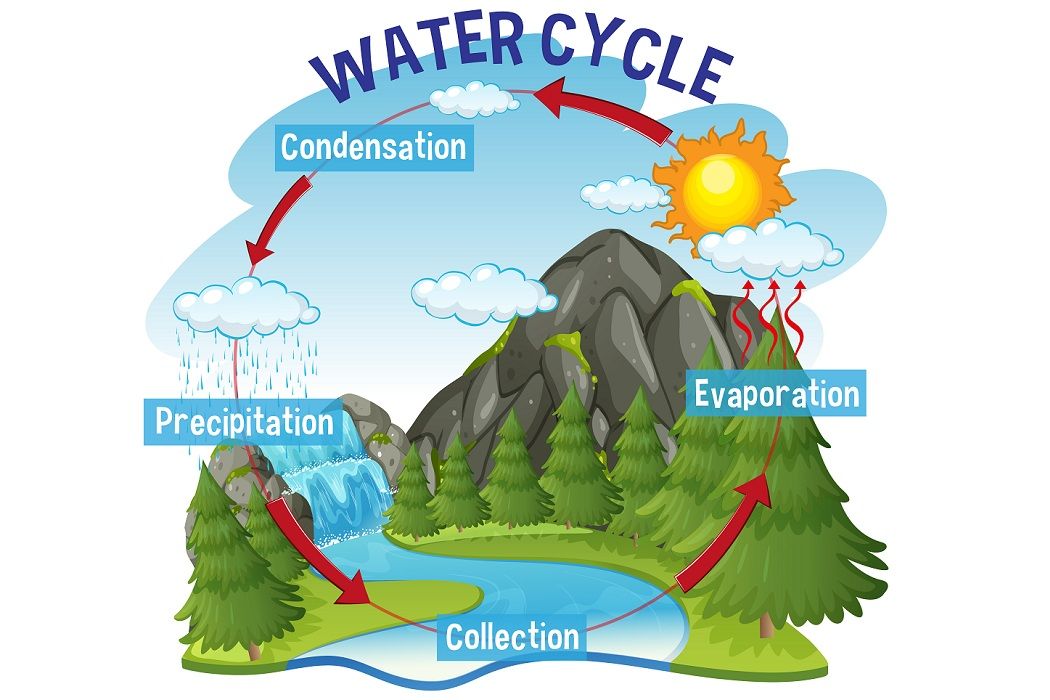
Hydrogen Bond Examples in Chemistry
If you are also interested in the other intermolecular forces van der Waals dispersion forces and dipole-dipole interactions , there is a link at the bottom of the page.
Hydrogen bonds have a longer bond length.
The strength of a typical hydrogen bond is about 5% of that of a covalent bond.
- Related articles
2022 mail.xpres.com.uy