Titration - Titration: Overview, Curves & Calculations
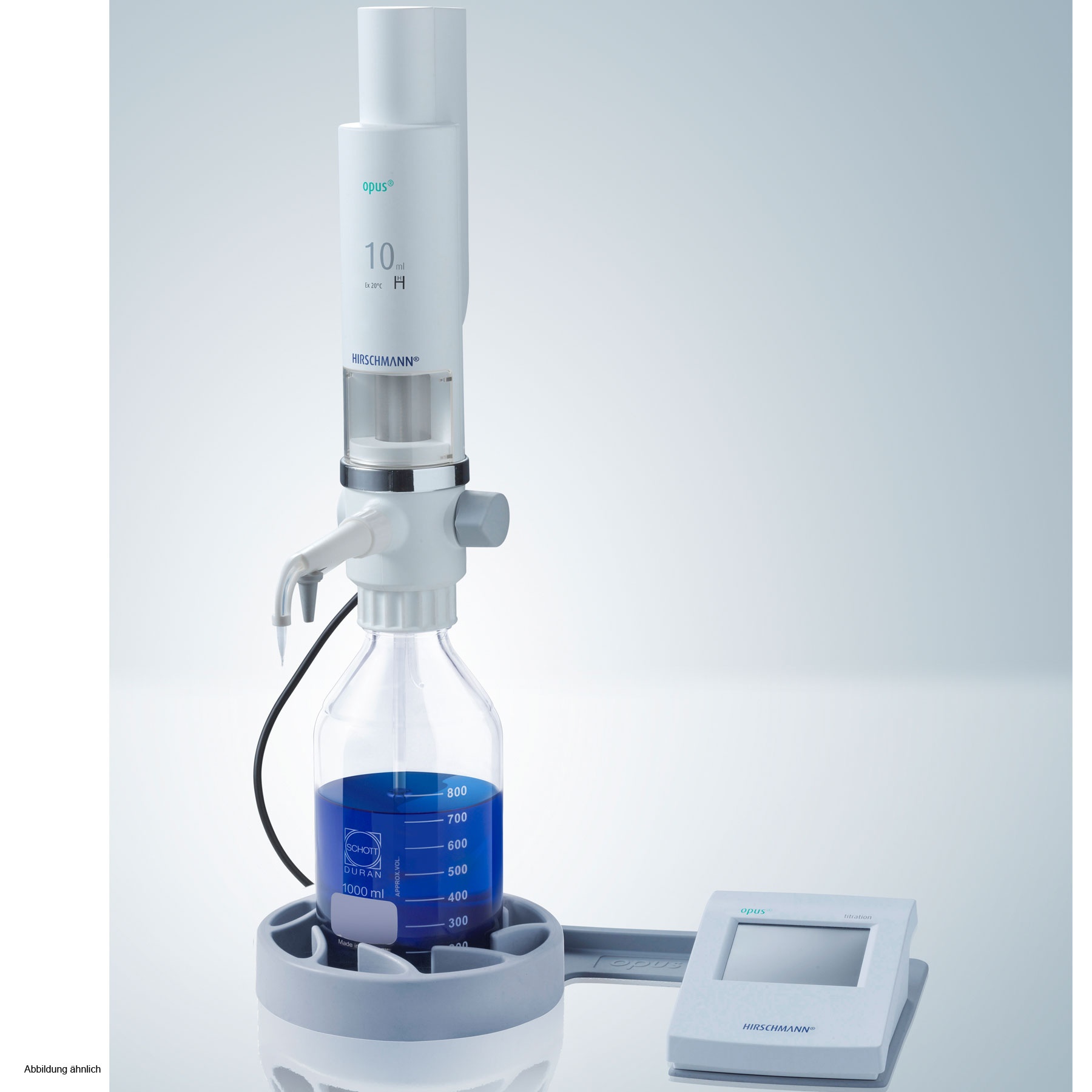
Titration: Principles, Volumetric Analysis
The procedure of titration completes when the colour of the indicator changes.
We can see the comparison of multiple tiration curves when using different weak bases with different p K b values.
The endpoint is not the equivalence point but a point at which the pH indicator changes color.
What Does ‘Titration’ Mean?
Swirl the water around your beakers and flasks a few times before draining it.
Even medical labs use this type of analysis to study blood and urine samples from patients.
We know that when a strong acid is placed in water, it will dissociate completely.
- Related articles
2022 mail.xpres.com.uy